New Breakthrough Enables Affordable, Eco-Friendly Hydrogen Production
By displacing pricey precious metal catalysts, a novel technique has been developed that makes it possible to produce green hydrogen more cheaply and sustainably, bringing society closer to becoming carbon neutral.
A collaborative research team led by Professors Dong-Hwa Seo of the Department of Materials Science and Engineering at KAIST and Jungki Ryu of the School of Energy and Chemical Engineering at UNIST has successfully developed a bifunctional water electrolysis catalyst for the high-efficiency and stable production of high-purity green hydrogen.
The recently designed catalyst shows remarkable stability even in extremely acidic and corrosive settings. The catalyst, which uses ruthenium, silicon, and tungsten (RuSiW) instead of traditional platinum (Pt) or iridium (Ir) catalysts, is more affordable. Moreover, it is an environmentally favorable substitute because it produces much less greenhouse gas.

Water electrolysis is a cutting-edge technology that produces hydrogen through the process of electrolyzing water. It is considered a key technology for achieving a carbon-neutral society as it enables the production of environmentally friendly hydrogen without carbon emissions.
The goal of the study team was to identify substitutes for precious metal catalysts, such as iridium and platinum, which show stability in acidic environments. Due to its comparatively low cost of manufacturing and much lower greenhouse gas emissions when compared to platinum and iridium, ruthenium has attracted attention as an environmentally benign metal. However, because of its inferior stability to iridium and poorer catalytic activity to platinum, it encountered difficulties in the commercialization process.
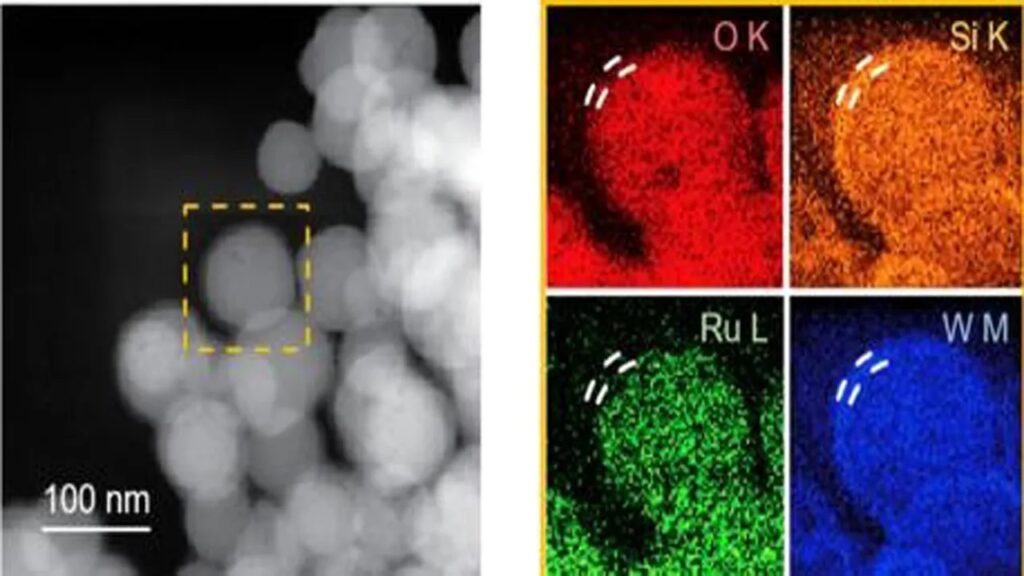
The study team created a catalyst based on ruthenium, silicon, and tungsten to get around these restrictions. The researchers showed the catalyst’s potential as a bifunctional catalyst by improving the performance of the ruthenium catalyst, which has lower stability in both the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER).
The created catalyst has a silicon and tungsten-doped structure centered on a ruthenium atom. By boosting the protons’ adsorption intensity on the catalyst surface, the catalyst’s capacity to accelerate reactions was improved. In the hydrogen evolution reaction, it is more active than platinum catalysts that are sold commercially. In addition, the stability of ruthenium is enhanced by a thin 5–10 nm-thick tungsten coating that shields the catalytic site.
The catalyst was the subject of a stability experiment by the study team. They introduced 10 mA of current into a 1 Ꭰ electrode using an acidic electrolyte (with an acidity of 0.3). The catalyst that was created exhibited consistent performance even after operating for more than 100 hours.
“This three-element catalyst’s development is significant because it has the potential to replace expensive platinum and iridium simultaneously,” Professor Ryu said. Since it can be produced readily and steadily even under extremely corrosive acidic circumstances, it is anticipated to be used in high-purity green hydrogen generation systems, such as PEM electrolyzers.
Source : https://onlinelibrary.wiley.com/doi/10.1002/adma.202304468




